Abstract
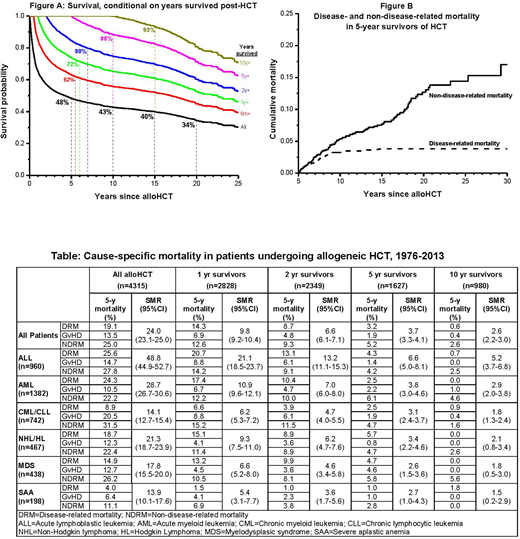
Background: alloHCT is offered with curative intent to patients with malignant as well as some nonmalignant hematologic diseases, and conventionally-computed survival estimates are offered for prognosticating outcomes. However, conventionally-computed survival and mortality risk estimates do not account for patients' elapsed survival time which, among other factors, could affect subsequent mortality. Conditional survival overcomes these limitations by calculating the probability of survival after having already survived a certain period of time - such data are unavailable for alloHCT recipients. We describe conditional survival and cause-specific mortality (disease-related [DRM], non-disease-related [NDRM], and GvHD-related) after alloHCT to provide clinically relevant information for patients who have survived 6 mos, 1, 2, 5, and 10y after alloHCT. Methods: From 1976 to 2013, 4,315 consecutive patients underwent alloHCT for hematologic diseases at a single institution. Vital status and cause of death were determined using the National Death Index Plus and medical records. Results: Diagnoses included acute leukemia (54%), chronic leukemia (17%), lymphoma (11%), myelodysplastic syndrome (10%), severe aplastic anemia (5%), and other hematologic diseases (3%); median age at HCT was 38.5y (0.3-75.4). As of December 31, 2014, 1841 patients were still alive in whom the median follow-up was 8.5y (0.2-36.6). Of 2,474 deaths (57% of cohort) for whom causes of deaths are available, 42% was due to primary disease, 30% to graft versus host disease (GvHD), 12% to infection, 5% to cardiopulmonary diseases, 3% to subsequent malignant neoplasm (SMN), and 8% to other causes. Conventionally-computed probabilities of survival at 5, 10, 15, and 20y after alloHCT were 48%, 43%, 40%, and 34%, respectively. On the other hand, for patients who had survived 6 mo, 1, 2, 5, 10y after alloHCT, 5-y conditional survival rates were 62%, 72%, 80%, 88% and 93%, respectively (Figure A). Overall, the cohort was at a 24-fold (Standardized Mortality Ratio [SMR]=24.1, 95%CI=23.1-25.0) risk of any death, compared to the general population; the risk of death from pulmonary complications was 31-fold, that from SMN was 31-fold, and that from cardiovascular complications was 3.5-fold. SMR and cause-specific conditional mortality rates by primary diagnosis are shown in the Table. Significantly elevated risk of all-cause mortality persisted in patients who survived 5 and 10y post alloHCT (SMR=3.7, 2.6, respectively, p<0.05), although DRM and GvHD-related mortality in the subsequent 5y was low (<6%). In comparison, NDRM increased over time; among 5y survivors, NDRM exceeded DRM (Figure B): SMN was the most common cause (34%), followed by GvHD (26%), other causes (15%), infection (14%), and cardiopulmonary disease (11%). In 10y-survivors of acute leukemia and chronic leukemia, all-cause mortality remained significantly higher compared to the general population (SMR>1.8, p<0.05). For the overall cohort, after adjusting for primary diagnosis, relapse risk at alloHct, treatment era, and ethnicity, DRM was significantly lower for patients who developed acute GvHD (HR=0.78, 95%CI=0.66-0.93). Adjusted for the same factors, NDRM risk increased with older age at HCT (HR=1.02 per year, 95% CI=1.01-1.03), and for patients with acute GvHD (HR=1.9, 95%CI=1.6-2.2) and those exposed to Total Body Irradiation (TBI) (HR=1.4, 95%CI=1.2-1.8). Conclusions: The projected 5-y survival rates improve conditional on time survived from alloHCT; 5-y survival is nearly 90% for those who have already survived 5y. However, alloHCT recipients who have survived 10+y continue to remain at increased risk of death compared to the general population, with SMN as the most common cause. Acute GvHD is associated with decreased risk of DRM as expected, whereas acute GvHD and TBI are associated with increased risk of NDRM. DRM and GvHD-related mortality rates decline with survival time, while among ≥5y survivors, NDRM exceeds DRM. Conditional survival and mortality estimates provide clinically relevant prognostic information, helping to inform preventive and interventional strategies.
Forman:Mustang Therapeutics: Other: Licensing Agreement, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal